A new corporate warranty role: the person responsible for compliance
The Article 15 of the Mdr introduces an important innovation: the obligation to appoint a person within the MD company who is responsible for compliance with the regulations (so-called Responsible Person or PR). The rule provides for this obligation on the part of the manufacturer (including micro/small enterprises) and also on the part of the authorized representative. More precisely, while the manufacturer will then have the obligation to identify the PR within his organization, for the authorized representative and the micro/small enterprises it will be sufficient to demonstrate that they have permanent and continuous access to it.
Assignments of the Person Responsible for Compliance
The inspiring reasoning of this new professional figure can be found in Recital 34 where it states: "It should be ensured that the supervision and control of the manufacture of devices, as well as post-market surveillance and surveillance activities related to them, are carried out within the manufacturer's organisation by a person responsible for compliance with the regulations and in possession of minimum qualification requirements".
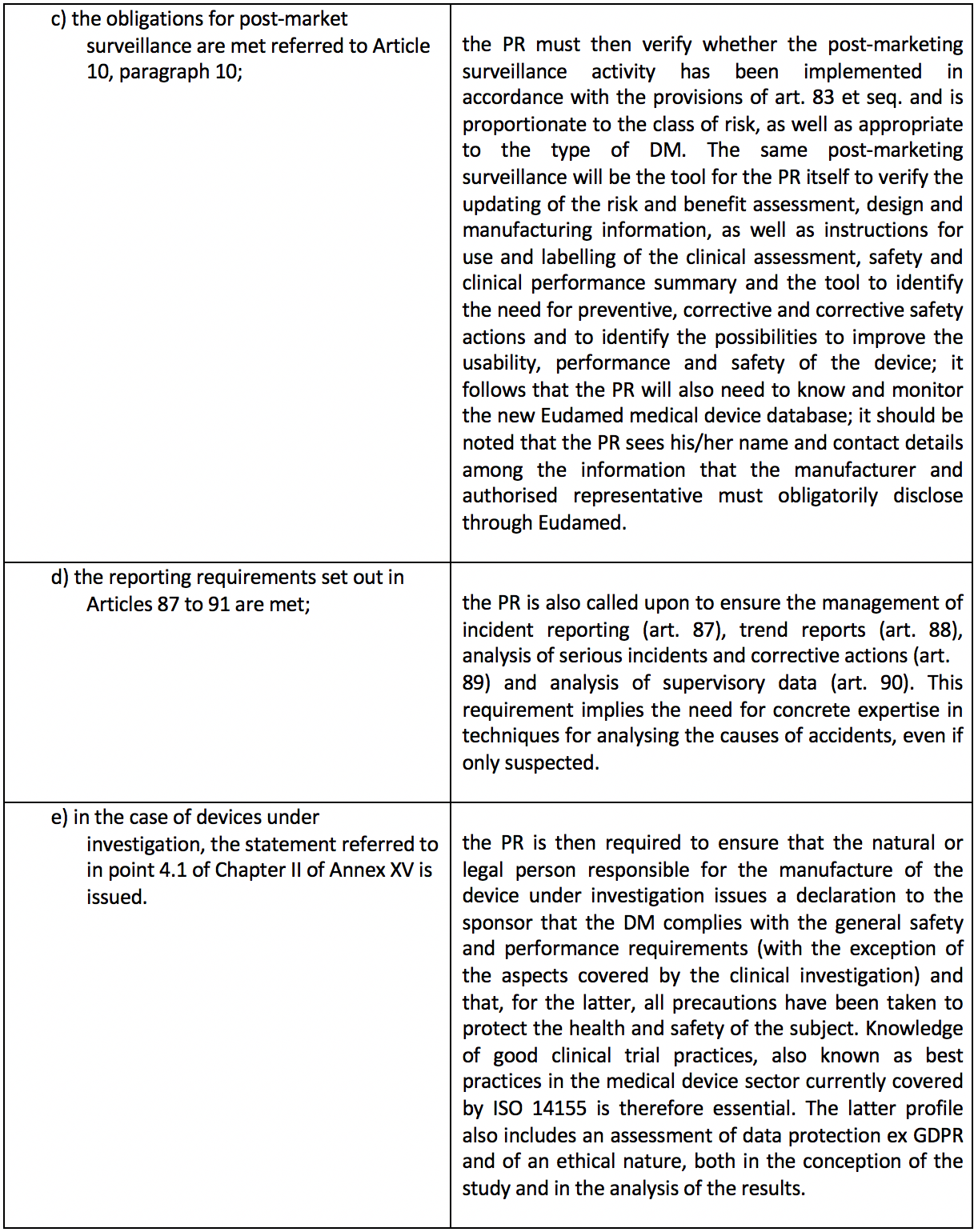
Basically, this is a kind of "internal guarantor" of a mandatory nature. The same Article 15 then lists in detail the tasks to be performed by the latter. For ease of reading, these tasks have been reported in the table below with a very brief commentary.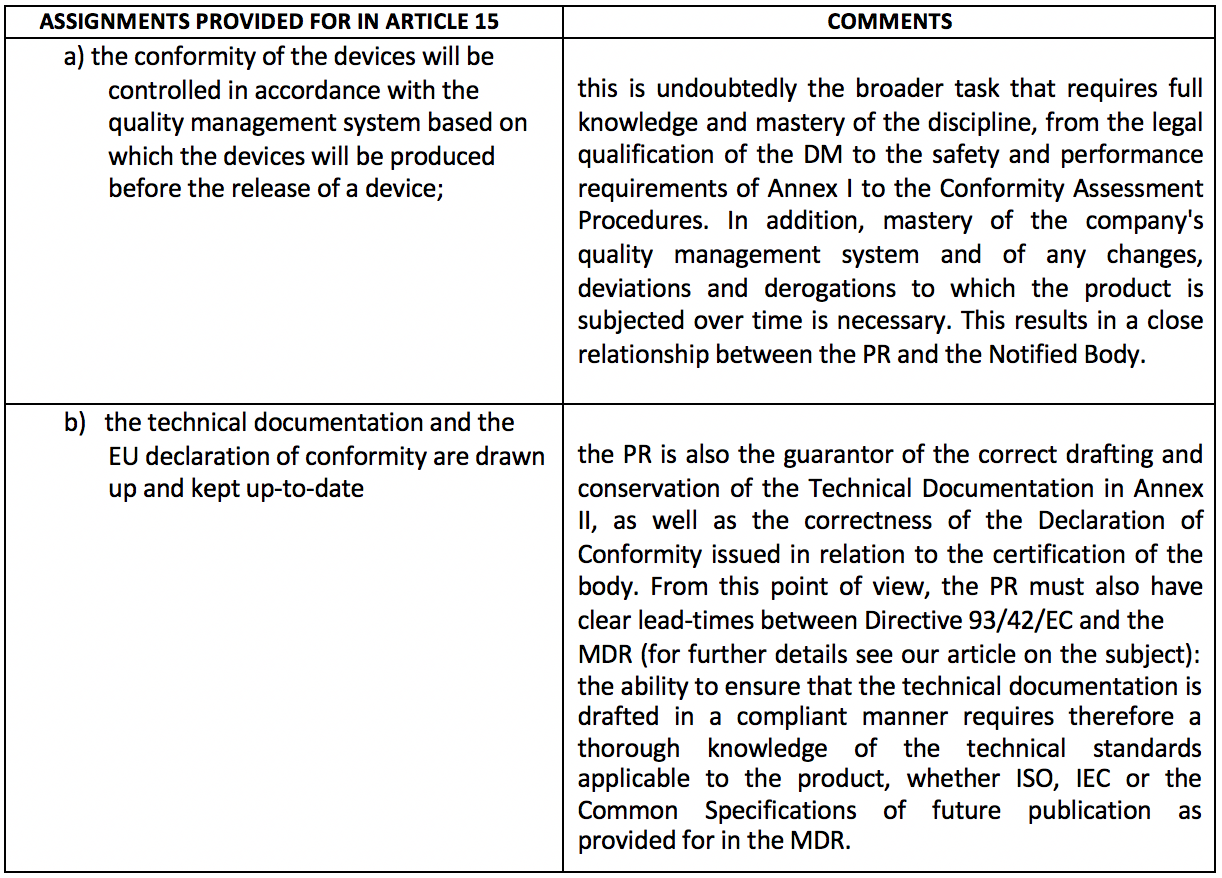
Training
Article 15 then sets out specific training requirements for the person wishing to take on the role of PR. More precisely, the rule also provides that this position may be taken on by a person with at least one of the following two alternative requirements.
- a diploma, certificate or other evidence of formal qualifications obtained on completion of university studies or a course of study recognised as equivalent by the Member State concerned, in Law, Medicine, Pharmacy, Engineering or another relevant scientific discipline, and at least one year's professional experience in the field of regulation or quality management systems relating to medical or
- four years' professional experience in the field of regulation or quality management systems relating to medical
Although the legislative provision provides for the possibility of an alternative between academic training (specific degrees) and training in the field (experience of at least 4 years), from the reading of the tasks it is clear that the skills of the PR will have to be much broader than a degree in Law or Pharmacy, ranging instead from legal knowledge and quality management system skills to specific knowledge of the substantive requirements of the DM. It is therefore clear that the European legislator has defined a much more articulated and multifaceted professional figure, called on the one hand to know the regulations but on the other to carry out much more complex and articulated management tasks.
Possible certifications
It is therefore necessary, both for professionals and companies, to provide concrete and measurable evidence of their specific skills and experience, both regulatory and sectorial with respect to the activity of the PR. To this end, although the regulations have not imposed the obligation for this professional figure to obtain a specific certification by a notified body, it may be important, also in light of the complexity of the task, the acquisition of a "voluntary certification" in order to see their attitudinal and professional skills recognized.
The certification of the PR- at the moment it is not accredited by the Italian Body Accredia - has been developed and it is issued by Aicq Sicev Srl (body accredited by Accredia in accordance with ISO/IEC 17024 for several certification schemes), which, in order to regulate the activity of the professional and identify a set of specific precepts aimed at contributing to the consolidation of the credibility of the national certification system, has drawn up a short code of professional ethics consisting of 16 articles.
The professional who has obtained the certificate "Responsible in compliance with medical devices regulations" will have the obligation to agree in writing with the manufacturer (or authorized representative) that outlines the obligations and responsibilities and, at the same time, include in the contract certain obligations provided for by the rules of professional ethics on good faith, fairness, dignity, decorum, partnerships, independence, professional training and guarantee of compliance with the DM. To these charges will be added, in addition to the prohibition to harm the health of patients and the image of the employer/committer, some communication obligations towards the management of Aicq Sicev (such as, for example, the occurrence of subjective and/or objective situations that make it excessively burdensome and/or impossible to perform the task properly, or the violation by the employer/committer of the rules relating to certification, marking or quality system).
Another important note consists in the fact that the violation of the rules of ethics will constitute a behaviour in contrast with the state of the PR that the Appeals Committee of Aicq Sicev may sanction with a written warning, suspension, or revocation of certification or, in extreme cases, with the removal of the professional from the register of "responsible in compliance with medical devices regulations".
Responsibility
It is necessary to understand what the responsibility profiles of the PR is. On this point, it should be pointed out that Article 15 does not establish anything about the fact that PR. must be a natural or a legal person: it follows that the appointment may be made in favour of both natural persons and companies, with the additional power to delegate, subject to written agreement on the respective area of competence, a plurality of subjects to compliance with regulatory compliance.
With regard to responsibility profiles, the following is noted.
Administrative responsibility
As things stand at present, no decree has yet been issued to adapt the Italian State to the new MDR. It is therefore not known whether or not administrative sanctions will be imposed on the PR. Even if - as is likely
- the PR is not directly sanctioned, this will not lead to a total exemption from liability: in fact, where the sanction as a result of a failure by the PR to comply with its duties, it may be sued in civil proceedings to return an amount equal to the damage caused "indirectly" to the manufacturer.
Such a hypothesis could occur in the following two circumstances:
- the sanction imposed is the consequence of a breach of an obligation expressly provided for in the contract by PR;
- the conduct of PR, although not corresponding to a contractual obligation, falls within the cases provided for by law to exercise the action of recourse and has caused the damage (or the imposition of the sanction).
Civil liability
With regard to liability, however, the situation is different depending on whether the PR is an employee of the manufacturer (or the authorised representative) or an external party.
In fact, where the professional is an employee of the structure, pursuant to the combined provisions of articles 2049 and 2104 of the Civil Code, recourse will be considered admissible in cases where the employee has not used the diligence required by the nature of the service or, in any case, has violated the instructions given by the employer for the performance and discipline of the work. In addition to this requirement, then, it will be the employer's responsibility to prove in court that he has put the employee in a position to carry out his duties correctly and that he has carried out the necessary checks on him.
If, on the other hand, the office of PR is held by a professional outside the organization or by a service company, the charge of liability, pursuant to article 1176 of the Italian Civil Code, may occur if the client proves that the professional has failed to exercise the diligence required by the nature of the assignment and, therefore, the culpable subjective element resulting from negligence, imprudence or inexperience. In order to clarify the above, it should be noted that, if the performance involves technical problems of special difficulty, article 2236 of the Italian Civil Code requires an additional element in addition to general negligence, constituted in such instances of malice or gross negligence.
For both liability profiles, in order to reduce/manage the legal risks, it will be necessary to draw up the contract taking care to delimit with great precision the obligations and responsibilities of both parties.