Apps and telemedicine in view of the new MDR
In this article published by Sergio Pillon (coordinator of the Joint Technical Commission of the Italian Ministry of Health for the development of national telemedicine) on the development of digital healthcare in Italy, the Israeli organization of healthcare is taken as an example, and it is stated that "digital healthcare is the backbone of the system, that is focused, from the patient's point of view, on services offered through apps and supported by Big Data and the network". I am of the same opinion. The use of software and apps in healthcare and the potentiality related to the processing of data collected through software, can lead to a significant change in the organization of our public and private health service. It must therefore be clear what legal changes will entail the new Mdr in the field of software and medical applications.
Key points to be analysed
First of all, it is necessary to establish whether a software/ app is a medical device or an accessory for a medical device, thus falling within the scope of application of the Mdr. And then determine which risk class should be applied in light of the new Regulation.
Legal qualification of software
The changes that are brought by the new Mdr in the field of software, have already been anticipated by the judgement of the Court of Justice of the European Communities of 7 December 2017 - C 329/16, which deserves a brief comment. The sentence originates from a dispute initiated by the French association Snitem (a medical technology company) during which the Court of Justice was asked to assess whether the software that has "at least one function that permits the use of data specific to a patient to help his doctor issue his prescription, in particular by detecting contraindications, drug interactions and excessive doses" should or should not be considered a medical device, taking into account that it is not used "in" or "on" the human body.
The Court - after recalling the judgement CGCE 22 November 2012 C-219/11 (so-called Brain Products case) in which the Court declared that a software is a medical device, only when it is intended for a medical purpose and not when it has research purposes - while acknowledging that article 1 lett. a) of Dir 93/42/EEC (applicable at the time) refers to a "use for human beings", has recalled Recital 6 of the Dir 2007/47, which states broadly "that software in its own right, when specifically intended by the manufacturer to be used for one or more of the medical purposes set out in the definition of a medical device, is a medical device. On this assumption, the Court stated that the software can be a medical device even if not employed "on human beings".
According to the judges of the Court then "the EU legislature intended to focus, in order to classify software as a medical device, on the purpose of its use and not the manner in which the effect it is capable of producing on or in the human body is likely to materialise". Consequently "it does not matter whether, in order to be classified as a medical device, software acts directly or indirectly on the human body, the essential point being that its purpose is specifically one of those referred to in the definition of medical device”.
The reinforcement of the new Mdr
This jurisprudential expansion of the notion is reinforced today in the new Mdr. Recital 19 establishes in fact that “it is necessary to clarify that software in its own right, when specifically intended by the manufacturer to be used for one or more of the medical purposes set out in the definition of a medical device, qualifies as a medical device, while software for general purposes, even when used in a healthcare setting, or software intended for life-style and well-being purposes is not a medical device. The qualification of software, either as a device or an accessory, is independent of the software's location or the type of interconnection between the software and a device”.
The notion of “accessory for a medical device”
Furthermore, the notion of "accessory for a medical device" in the Mdr is broader than the concept of accessory as used in Dir 93/42/EEC. Article 2 letter 2 provides that an “‘accessory for a medical device’ means an article which, whilst not being itself a medical device, is intended by its manufacturer to be used together with one or several particular medical device(s) to specifically enable the medical device(s) to be used in accordance with its/their intended purpose(s) or to specifically and directly assist the medical functionality of the medical device(s) in terms of its/their intended purpose(s)”.
In fact, the first part of the article follows the previous notion of accessory (Article 1 letter b), while the second part of the standard extends the notion of an accessory also to all products that “assist the medical functionality of the medical device(s) in terms of its/their intended purpose(s)”. Thus, there will be an increase in products - especially software - that will be qualified as accessories for medical devices, to which, consequently, the new Mdr will be fully applicable.
Risk class
The number of software/apps that will have to be included in the legal notion of medical device/ accessory will certainly increase, but risk class of the software may rise as well.
In fact, while today most of the software (and most of the apps) fall into the risk class I (affixing of the CE marking without the intervention of the Notified Body) the Rule 11 of the MDR establishes very different rules, that are summarized in the table below.
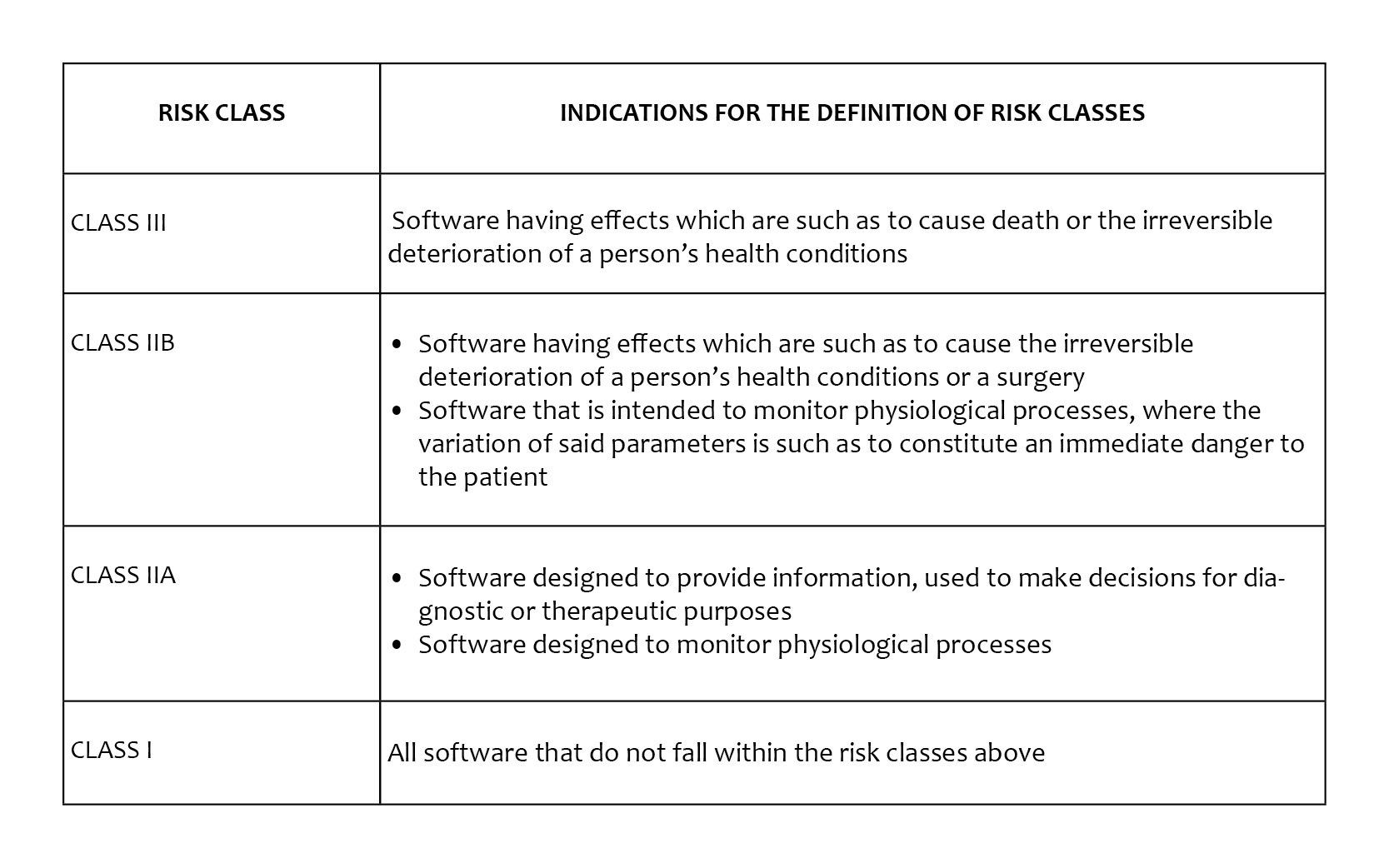
The table above clarifies that software that monitors physiological processes or that in any case provides the doctor with information used to make diagnostic or therapeutic decisions (which is a really broad provision) will fall into class IIA or IIB, with consequent involvement of the Notified Body. Furthermore, according to rule 3.3 "the software intended to operate a device or to influence its use, falls within the same class as the device". It is therefore undeniable that the legal framework has strongly changed, which will have a significant impact on the market, also from an economic point of view.